Informed Consent Example – A Comprehensive Guide for Students and Researchers
In the field of academic research, especially in medical, psychological, and social science studies, the principle of informed consent plays a critical role in protecting the rights and dignity of participants. If you’re a student or researcher preparing a research proposal or conducting human-based research, understanding how to properly design and implement informed consent is essential. In this post, IvyResearchWriters.com offers you a comprehensive guide to the informed consent process and presents informed consent example templates that meet ethical standards.
What Is Informed Consent?
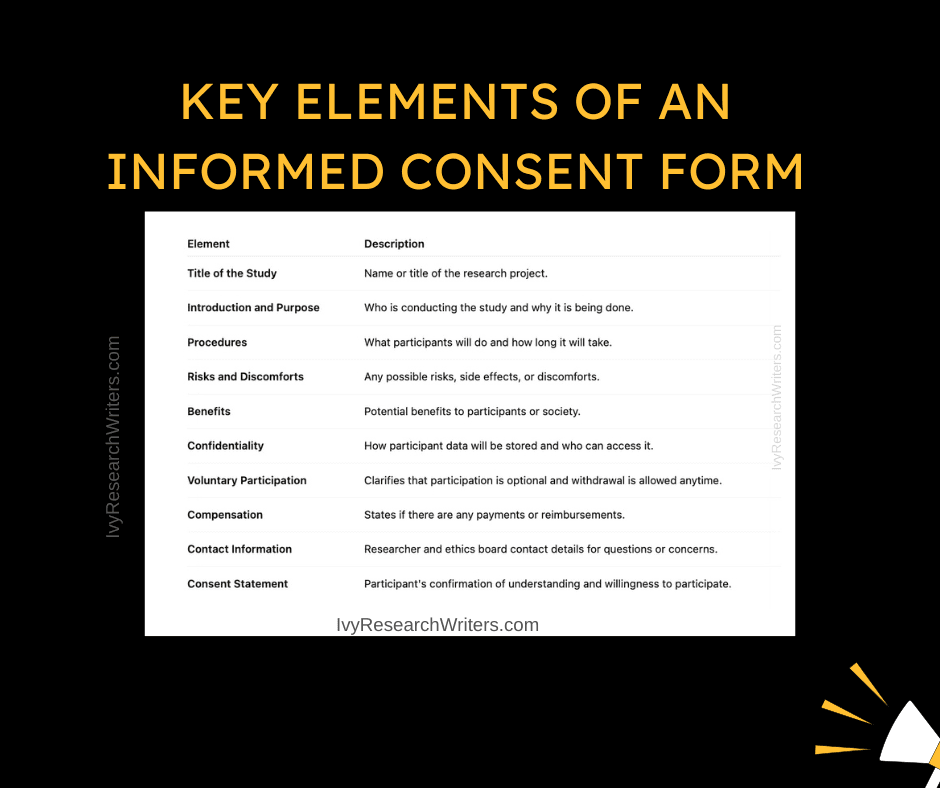
Informed consent is a formal agreement in which a participant voluntarily confirms their willingness to take part in a study after being informed about all relevant aspects of the research. This includes understanding the study’s purpose, procedures, potential risks, benefits, confidentiality, and the right to withdraw without penalty.
Key Components of Informed Consent:
- Disclosure – All necessary information must be provided clearly.
- Comprehension – Participants must fully understand what they are agreeing to.
- Voluntariness – Participation must be free from pressure or coercion.
- Competence – The individual must be mentally capable of making the decision.
- Consent – A documented or verbal agreement must be obtained.
Why Is Informed Consent Important in Research?
Informed consent is not just a formality; it is a legal and ethical requirement. It ensures the autonomy and protection of research subjects, particularly when the research involves vulnerable populations such as minors, the elderly, or those with limited cognitive abilities.
At IvyResearchWriters.com, our experts ensure that your research project includes a well-drafted informed consent form that meets institutional review board (IRB) guidelines and international ethical standards.
Informed Consent Example: Medical Research
Below is a simplified informed consent example used in clinical or biomedical research:
Title of Study: The Effect of Sleep Duration on Cognitive Performance in Adults
Researcher: Dr. Jane Smith, University of Health Sciences
Purpose of the Study: To investigate how different sleep patterns affect memory and attention in healthy adults.
Procedures: Participants will be asked to complete memory tests and wear a sleep-monitoring device for one week.
Risks: Minimal discomfort due to sleep monitoring.
Benefits: Contributing to scientific knowledge about sleep and cognition.
Confidentiality: Your data will be stored securely and used only for research purposes.
Voluntary Participation: You may refuse to participate or withdraw at any time.Consent Statement:
I have read and understood the information above. I understand that my participation is voluntary, and I can withdraw at any time. I agree to take part in this research study.Signature of Participant: _____________________ Date: __________
Signature of Researcher: _____________________ Date: __________
Informed Consent Example: Social Science Research
For non-clinical studies, such as in psychology or education, the consent form is more straightforward:
Title of Study: Exploring the Relationship Between Study Habits and Academic Performance in High School Students
Researcher: Mark Allen, Graduate Student, Department of Education
Purpose: To study how different study techniques impact grades among students.
What You Will Do: Fill out a questionnaire and participate in a one-hour interview.
Risks: None identified.
Confidentiality: Responses will be anonymized.
Voluntary Nature: You may skip any question or leave the study at any time.Consent:
I voluntarily agree to participate in this study.Signature of Participant: _____________________ Date: __________
How to Write an Informed Consent Form
If you’re preparing a form for your academic or institutional research, follow these steps:
- Use simple, clear language that is understandable to a layperson.
- Avoid jargon or complex medical terms.
- Organize the form into sections: purpose, procedure, risks, benefits, confidentiality, voluntary participation, contact info, and signature fields.
- Include version numbers and dates to track updates.
- Consult your institution’s ethics committee or IRB for templates and guidelines.
Informed Consent for Minors and Vulnerable Populations
In cases involving children or individuals with limited capacity, parental or guardian consent is required. Often, assent from the child is also requested. Researchers must be extra careful to ensure comprehension and protect participants’ welfare.
Example:
Parental Consent Form for Minors in Educational Research
I give permission for my child to participate in the study titled “Learning Strategies and Student Engagement.” I understand the research is voluntary and confidential.Parent/Guardian Signature: __________________ Date: ________
Child Assent (if applicable): ________________ Date: ________Ready to ensure ethical compliance in your study?
Get our professionally crafted Informed Consent for Minors and Vulnerable Populations form and safeguard participant rights with confidence.
Hire Research Writer
Tips for Implementing Informed Consent in Your Research
- Always document the consent process. Use digital or physical records as proof.
- Offer participants a copy of the consent form for their own records.
- Train your research team on how to explain consent clearly and respectfully.
- Respect the right to withdraw, even if the study is ongoing.
At IvyResearchWriters.com, our research assistance services include drafting and reviewing informed consent forms that meet IRB and university research standards. Whether you are working on a minor research project, thesis, or major scientific study, we provide ethical writing support tailored to your field.
Common Mistakes in Informed Consent
- Using overly technical language.
- Failing to mention the right to withdraw.
- Not clarifying data confidentiality.
- Skipping consent for secondary data use.
- Collecting signatures without proper explanation.
Avoid these errors by consulting professional writing support like IvyResearchWriters.com.
What are Informed Consent Guidelines?
Informed consent guidelines are a cornerstone of ethical healthcare practice, ensuring that patients can make informed decisions regarding their treatment. There are 3 types of informed consent in healthcare: verbal, written, and implied. A general requirement for the informed consent form is that it includes key elements such as potential risks and benefits, ensuring compliance with HIPAA and other professional guidelines.
In certain situations, a criterion for waiving informed consent is that when appropriate, such as in emergency settings. Obtaining informed consent also involves assessing consent elements for counseling informed consent, consent to disclose medical information, and consent to release information, including forms like the consent to disclose medical information NYC form. Furthermore, advance informed consent to dual agency with designated sales agents must adhere to research ethics review and the common rule.
Healthcare providers must ensure that patients understand the elements of informed consent to uphold the highest ethical standards. This includes providing consent for release of financial aid information and facilitating informed decision-making. The code of ethics mandates that consent must be obtained in a manner that respects patient accessibility and autonomy, ultimately supporting their right to make healthcare decisions.
Summary: Diverse Examples Ensure Ethical Research Practices
Ensuring ethical research practices involves multiple examples of informed consent that highlight the importance of consent to release information form. To define informed consent, it is essential to understand the definition for informed consent, which includes elements of an informed consent. For instance, Erin’s informed consent map provides an example of informed consent that is inclusive and mitigates misunderstandings throughout the process.
Moreover, examples informed consent form templates can be found through resources like Health and Human Services or the FDA guidance informed consent. When involving human participants, your informed consent form must describe the subject population and any potential risks. Written consent and verbal consent are both ethical and legal requirements, with some jurisdictions allowing for a waiver under specific conditions.
In counseling, examples of informed consent in counseling can be structured to ensure that clients fully understand their rights and the implications of disclosing personal information without consent is a serious breach of trust. Templates developed by institutions like Fordham University parental consent for exchange of information can further guide researchers in obtaining free prior and informed consent.
Ultimately, maintaining an ethical approach in research is an ongoing process that requires consultation with committee members and adherence to Wisconsin informed consent laws, ensuring that subjects are adequately informed and protected throughout the research journey.
Informed Consent Process
Informed consent is a crucial process in healthcare and research, defined as the voluntary agreement of an individual to participate in an intervention after being fully informed. An informed consent example would include a detailed informed consent document template outlining risks and benefits. Informed consent for research differs from implied consent, as the former requires explicit agreement. The purpose of informed consent is to ensure ethical practice, protecting individuals from non informed consent.
Organizations like the Informed Consent Action Network advocate for clear understanding, emphasizing the four principles of informed consent: autonomy, beneficence, non-maleficence, and justice. Informed consent definition psychology highlights the importance of mental competencies in the process. For example, an informed consent form example for therapy or HRT should be accessible at an appropriate reading level to ensure comprehension, ethically guiding individuals through their decisions.
In clinical settings, the informed consent for counseling sample serves as a vital tool for transparency, while sharing medical information without consent is strictly prohibited. The informed consent definition emphasizes the need for a thorough assessment of understanding, ensuring that patients and participants are well-informed before proceeding. Physicians for informed consent stress the necessity of a sample informed consent form that caters to individual circumstances, promoting ethical and preventive healthcare practices.
Informed consent documents
Informed consent is a crucial element in healthcare and research, particularly in clinical trials. An informed consent example might include a sample informed consent document detailing the risks and benefits of participation. The purpose of informed consent is to ensure that participants understand their involvement, which aligns with the belmont principle of respect for persons. Informed consent in the context of therapy or HRT emphasizes the need for clear communication between physicians and patients.
Moreover, informed consent in research with homeless youth highlights the importance of understanding the ethical implications of participation. Informed consent meaning extends to differentiating implied consent vs informed consent, where the latter requires explicit agreement after adequate information is provided. For example, an informed consent counseling template can guide mental health professionals in obtaining informed consent in psychology, ensuring that clients are fully aware of their rights and the treatment process.
What is informed consent in healthcare
In healthcare, informed consent is a critical process that ensures patients are fully aware of the implications of their medical decisions. For instance, an example of an informed consent would involve a physician outlining the risks and benefits of a procedure. The informed consent clinical trials definition emphasizes the importance of transparency in research, particularly in informed consent research involving vulnerable populations like homeless youth, where informed consent in research with homeless youth is often exempt.
The purpose of informed consent is to empower patients by providing them with necessary information, adhering to the informed consent requirements ema, which include a clear informed consent form counseling. Additionally, informed consent hrt pertains to hormone replacement therapy discussions. In psychology, what is informed consent in psychology? It is about ensuring that clients understand the therapeutic process, aligning with the informed consent is considered an application of which Belmont principle: respect for persons, one of the four principles of informed consent.
Informed consent is also a concise mechanism by which patients can exercise autonomy. For a valid informed consent form, it must describe specific requirements, such as the nature of the treatment, potential risks, and alternatives. Physicians must ensure that physicians informed consent discussions are clear and informative. Ultimately, which statement is correct about informed consent can be answered by considering that it is an essential aspect of ethical medical practice.
Informed consent psychology example
Informed consent def refers to the process by which individuals voluntarily agree to participate in research or therapy after being fully informed of its risks, benefits, and alternatives. Informed consent is an important outcome of what principle? It stems from the principle of autonomy, acknowledging participants’ rights to make informed choices. In informed consent psych, a typical informed consent sample outlines the necessary information for participants.
The purpose of informed consent is to ensure that individuals understand what they are agreeing to, protecting their rights and wellbeing. What are the 4 principles of informed consent? These include information disclosure, comprehension, voluntariness, and competence. What is an informed consent? It is a document affirming that participants are aware of the study details. Your informed consent form must describe the nature of the study, potential risks, and benefits.
Informed consent example counseling
The purpose of informed consent is: to ensure that clients understand the nature of the counseling process and agree to participate willingly. What is the informed consent in this context? It is a formal agreement that outlines the therapist’s approach, confidentiality, and the client’s rights. Your informed consent form must describe the potential risks and benefits of therapy, and the limits of confidentiality. Regarding informed consent in the U.S, it is a critical ethical requirement in mental health practice.
Standard Written Informed Consent Form
Informed consent is a crucial element in various fields, including medical and psychology. The definition of informed consent in clinical trials involves ensuring participants understand the risks and benefits of their involvement. An informed consent example in counseling often includes a template that outlines procedures, while informed consent therapy emphasizes client autonomy and understanding.
The purpose of informed consent is to respect participants’ rights, embodying the Belmont principle of respect for persons. Informed consent in research with homeless youth addresses unique challenges, ensuring ethical standards are upheld. Informed consent requirements may vary, but it is fundamentally about transparency and respect, as seen in informed consent medical practices.
Informed consent is also applicable in HRT and other medical interventions, where patients must be fully aware of their options. An informed consent example in psychology illustrates how practitioners engage clients in understanding their treatment. Overall, informed consent remains a critical outcome of ethical principles in both research and clinical settings.
Informed Consent in Clinical Trials
Informed consent in clinical trials is a pivotal process, ensuring that participants understand the nature and implications of their involvement. An informed consent example might include counseling sessions where potential participants review an informed consent form detailing risks and benefits. For instance, in studies involving hrt, the informed consent medical definition emphasizes participants’ understanding of hormonal treatments before enrollment.
Informed consent is considered an application of the Belmont principle of respect for persons, which highlights the ethical obligation to honor individual autonomy. In informed consent psychology, the informed consent requirements vary, especially in vulnerable populations, such as homeless youth. Research on informed consent demonstrates that the purpose of informed consent is to facilitate understanding and voluntary participation.
What does informed consent mean? It encompasses the process of providing comprehensive information, ensuring participants can make educated decisions. A sample informed consent template often outlines the study’s purpose, risks, and benefits, while physicians must prioritize informed consent in their practice. Ultimately, informed consent is integral to ethical research and healthcare, safeguarding participants’ rights and wellbeing.
Final Thoughts
Understanding and implementing informed consent is a fundamental part of responsible and ethical research. It not only safeguards participants but also enhances the credibility and quality of your research findings. By reviewing informed consent examples and applying best practices, you can ensure that your study meets the highest academic and ethical standards.
Need help writing a research paper with informed consent documentation? Our expert writers are ready to assist you. Whether you’re preparing for a minor thesis, clinical project, or a full-scale research paper, IvyResearchWriters.com offers tailored, confidential, and ethical research writing support.
Ready to Ensure Your Research Is Ethical and IRB-Approved?
Get professional assistance with your research proposal and informed consent forms at IvyResearchWriters.com. Contact us now to speak with our expert academic writers and elevate your research!
FAQs about Informed Consent Guidelines Template: Research Ethics for Psychologists
What is Informed Consent in Research Ethics?
Informed consent is a fundamental principle in research ethics, ensuring that participants are fully aware of the nature, risks, and benefits of their involvement in a study. It involves providing potential participants with comprehensive information about the research project, allowing them to make an informed decision about their participation. This process is critical in maintaining the ethical standards set forth by the APA and is often a requirement in human subjects research.
What are the Components of Informed Consent?
The components of informed consent typically include: 1. A clear explanation of the research purpose and procedures. 2. Information on the risks and benefits of participation. 3. Assurance of confidentiality and data protection. 4. The voluntary nature of participation, including the right to withdraw at any time without penalty. 5. Contact information for any questions or concerns. These elements ensure that participants have a thorough understanding of what they agree to, which aligns with informed consent requirements.
What are the Types of Informed Consent?
There are generally three types of informed consent in healthcare and research: 1. Written Consent: A signed document that outlines all aspects of the study. 2. Verbal Consent: A spoken agreement often used when the research is less invasive and poses minimal risk. 3. Implied Consent: Typically applies in situations where participation is evident through actions, such as filling out a survey. Each type serves different scenarios and must comply with relevant informed consent guidelines.
What is the Role of an IRB in Informed Consent?
An IRB (Institutional Review Board) plays a crucial role in overseeing the ethical conduct of research involving human subjects. Before any research project begins, the IRB reviews the proposed studies to ensure that the informed consent process is adequate and that the rights and welfare of participants are protected. This includes ensuring that consent forms meet the clinical trial informed consent requirements and that risks are minimized.

